Knowledge your consolation stage with technological know-how will help employers gauge how immediately you might adapt to the specific methods applied of their facilities And the way successfully you may execute your responsibilities.
Calibrated gear offers precise measurements, important for maintaining the consistency of our products and solutions. It ensures that we meet regulatory specifications and customer anticipations.
To this question, you should generally reply that you will be very aware of the working day time desk of a pharmaceutical salesman.
Finally, it aids in traceability. In case of product remembers or investigations because of adverse functions, exact and detailed documentation may also help establish the root bring about quickly and successfully.”
We note that FDA published assistance detailing the problems below which FDA does not intend to get action when certain biological solutions are blended, diluted, or repackaged in a way not described within their permitted labeling.
“In taking care of cross-useful groups inside of a pharmaceutical engineering environment, I center on clear interaction and aim alignment. It’s very important to make certain Every person understands the undertaking goals, their purpose, And exactly how they lead to the general results.
A: Guides that provide a substantial stage beginning assurance that a specific system process or system regularly produces results that match designated conditions.
Furthermore, I made absolutely sure personal protective equipment was readily available and made use of correctly. Gear upkeep schedules had been strictly followed to avoid malfunctions that might cause incidents.
From the pharmaceutical sector, continual Studying is very important as a result of evolving technologies and laws. Therefore, I inspire a culture of ongoing education and learning and Experienced advancement amid my group customers.”
What's more, I am Component of quite a few professional networks in which we share insights and here go over modern developments. On the web platforms like LinkedIn are perfect for this function as well.
I’ve utilized QbD rules in procedure style and design and optimization. This associated pinpointing significant high quality attributes and defining design and style space employing possibility evaluation applications like FMEA.
Away from Specification (OOS) effects are those results, generated for the duration of screening that do not adjust to the relevant specification or expectations or While using the outlined acceptance requirements.
Our pharmaceutical consulting Authorities will develop a personalized tactic according to your products and organization’s individual needs. Our regulatory compliance consumers include:
This calendar year on the convention I used to be aiming to broaden my know-how and improve my greatest techniques for professional medical know-how conferences get more info which have HCPs in attendance. Though here, I've discovered about some changes, read some amazing results tales and received a great deal of sources but I have also acquired that my company, particularly our internal meeting organizing crew is undertaking an excellent career and suitable heading in the right direction With regards to becoming compliant!
 Alicia Silverstone Then & Now!
Alicia Silverstone Then & Now! Marla Sokoloff Then & Now!
Marla Sokoloff Then & Now!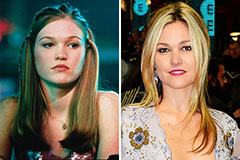 Julia Stiles Then & Now!
Julia Stiles Then & Now!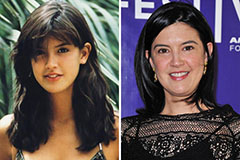 Phoebe Cates Then & Now!
Phoebe Cates Then & Now! Daryl Hannah Then & Now!
Daryl Hannah Then & Now!